
Laura M. Kidd Cordova
Practice Areas
Experience
- Employment & Governmental Investigations
- Export Controls Enforcement
- Global Anti-Corruption Compliance and Defense
- Global Corporate Victim Advocacy
- Government Contracts & Procurement
- Healthcare Fraud & Abuse
- Healthcare Litigation
- Healthcare Regulatory Compliance
- Investigations & White Collar Defense
- Securities Enforcement
- Special Investigations
Biography
A trial attorney and former federal prosecutor, Laura Cordova draws on her experience to effectively represent individuals and companies in federal criminal and civil government enforcement actions and related complex litigation. Laura’s practice includes handling sensitive internal investigations, navigating high-stakes government investigations, and defending clients in complex criminal and civil litigation. Laura has broad experience representing clients in criminal and civil fraud cases and investigations.
While at DOJ in Washington, DC, Laura supervised healthcare fraud prosecutions around the country and led the Corporate Healthcare Fraud Strike Force. In addition to her supervisory role at DOJ, Laura investigated and tried numerous complex cases, involving healthcare fraud, violations of the Anti-Kickback Statute, mail and wire fraud, money laundering, false statements, and government program fraud. Laura has extensive experience representing clients across the healthcare industry in criminal and False Claims Act matters involving allegations of healthcare fraud, Anti-Kickback Statute violations, and procurement fraud.
In addition to her legal practice, Laura served as an adjunct professor of law at the University of Houston Law Center, where she taught a course on health care fraud and abuse.
Education
B.S., Texas A&M University
Master of Public Health, Johns Hopkins Bloomberg School of Public Health
J.D., Georgetown University Law Center
Bar Admissions
Texas
District of Columbia
New York
Court Admissions
U.S. District Court for the Southern, Northern, Eastern, and Western Districts of Texas
U.S. District Court for the Eastern and Southern Districts of New York
U.S. District Court for the Eastern District of Wisconsin
U.S. Court of Appeals for the Fifth Circuit
- Represented social media influencer charged with securities fraud—all charges dismissed
- Represented foreign-exchange trader charged with wire fraud for allegedly “front-running” a foreign exchange transaction—all charges dismissed
- Represented CEO in DOJ and SEC securities fraud investigations—no charges brought
- Represented pharmacy chain in DOJ investigation related to allegations of improperly obtained prior authorizations and violations of the Anti-Kickback Statute—no charges brought
- Represented trade organization in DOJ investigation related to health insurance premium assistance programs—no charges brought
- Represented nonprofit in DOJ investigation related to pharmaceutical patient assistance programs—no charges brought
- Represented executive in DOJ investigation related to drug pricing and alleged Anti-Kickback Statute violations—no charges brought
- Represented pharmaceutical sales representative in DOJ investigation related to alleged off-label marketing and violations of the Anti-Kickback Statute—no charges brought
- Represented hospital system in DOJ and state Attorney General investigations related to tax credits—no charges brought
- Represented global agricultural commodity trading company in criminal DOJ and USDA investigation related to imported agricultural products—no charges brought
- Conducted internal investigation for national hospital system in response to internal whistleblower allegations of Anti-Kickback Statute violations and improper patient inducement, and advised system regarding disclosure obligations and potential FCA liability
- Conducted internal investigation related to billing code errors, and advised regional hospital system regarding disclosure obligations and potential FCA liability
- Conducted internal investigation and advised healthcare products distribution company regarding compliance, disclosure obligations, and potential FCA liability related to various federal government contracts, including pricing obligations, the Buy American Act, and the Trade Agreements Act
- Conducted internal investigation and advised health plan regarding compliance, disclosure obligations, and potential FCA liability related to California state contract requirements
- Chambers USA: America’s Leading Lawyers for Business, Ranked for Litigation: White-Collar Crime & Government Investigations (Texas), 2025
- The Best Lawyers in America (Woodward/White Inc.), Commercial Litigation; Criminal Defense: White-Collar, 2023-2025
- Lawdragon 500 Leading Litigators in America, 2023-2025
- Washington DC Super Lawyer, Super Lawyers by Thomson Reuters, 2020-2021
- Assistant Attorney General’s Award for Distinguished Service, 2015
- Health and Human Services Inspector General’s Award for Excellence in Fighting Fraud, Waste, and Abuse, 2016
- IRS Criminal Investigation’s Recognition for Outstanding Support of the IRS Criminal Investigation Mission, 2016
- FBI Director’s Certificate for Demonstrated Excellence in Successfully Prosecuting a Major Criminal Case, 2014
- Speaker, “False Claims Act Developments,” UT Law 10th Annual Government Enforcement Institute (September 2024)
- Speaker, “Cross Border Privilege Issues,” 10th Annual Southeastern White Collar Crime Institute (September 2024)
- Speaker, “Criminal and Civil Enforcement and COVID Fraud,” 34th Annual National Institute on Health Care Fraud (May 2024)
- Author, “US Supreme Court to Decide Whether the Government Can Prove Knowledge When the Defendant’s Regulatory Interpretation Is “Objectively Reasonable”,” Jackson Walker Insights (January 2023)
- Author, “U.S. Supreme Court to Decide Standard for DOJ Dismissal of Qui Tam Cases,” Jackson Walker Insights (June 2022)
- Speaker/Moderator, “Litigation Challenges in Procurement Fraud Matters,” 10th Annual False Claims and Qui Tam Trial Institute (April 2022)
- Author, “What to Know About the Department of Justice’s Nationwide Crackdown on Healthcare Fraud,” Jackson Walker Insights (September 2021)
- Speaker, “Healthcare Enforcement and Why Effective Corporate Compliance Matters,” International Association of Defense Counsel (IADC) 2020 Midyear Meeting (February 2020)
- Speaker, “Healthcare Fraud Enforcement and Corporate Compliance,” 2019 National Asian Pacific American Bar Association (NAPABA) Convention (November 2019)
- Author, “DOJ Moves to Dismiss 10 Kickback-Related False Claims Act Complaints Against Pharmaceutical Companies,” Pratt’s Government Contracting Law Report (April 2019)
- Author, “DOJ Resets Bar for Corporate-Cooperation Credit and Shifts Emphasis in False Claims Act Cases from Individual Accountability to Monetary Recovery,” Westlaw Journal’s Government Contract (January 2019)
- Speaker, “801 Recent Developments in Individual Criminal and Civil Culpability,” Health Care Compliance Association’s (HCCA) 4th Annual Healthcare Enforcement Compliance Conference (November 2018)
- Author, “Are Government and Health Care Contracts the Exception That Swallows the Brand Memo’s Rule on FCA Enforcement?” Westlaw Journal’s Government Contract (October 2018)
- Author, “Department of Justice Leadership Previews Reforms to False Claims Act Enforcement: Significant Incentives for Cooperation and Strong Compliance,” Pratt’s Government Contracting Law Report (October 2018)
- Speaker, “Developments in Individual Criminal and Civil Culpability Post-Yates Memorandum,” American Health Law Association (AHLA) Fraud and Compliance Forum (September 2018)
- Speaker, “Deep-Dive into Reverse False Claims: Preparing for the Future of False Claims for MCOs,” American Conference Institute (ACI) Managed Care Disputes and Litigation Conference (May 2018)
- Speaker, “Pricing Governance and Anticipating Fraud and Abuse Risks in Light of the Current Pricing Debate,” American Conference Institute (ACI) 18th Annual Conference on Fraud and Abuse (March 2018)
- Quoted, “Ex-Fed. Health Care Prosecutor Laura Cordova Discusses Whistleblower Suits, Their Future Under Trump,” Corporate Counsel (March 2017)
- Speaker, “Expectations of the DOJ,” Society of Corporate Compliance and Ethics (SCCE) Boston Regional Compliance & Ethics Conference (March 2017)
- Speaker, “Developments with the False Claims Act and Qui Tam Enforcement in Light of the Escobar Decision,” American Conference Institute (ACI) 17th Annual Conference on Fraud and Abuse in the Sale and Marketing of Drugs, Devices, and Medical Technology (March 2017)
- Speaker, “A Spotlight on Recent Trends in Enforcement,” CBI’s 5th Annual Compliance Monitoring Forum (February 2017)
- Speaker, “Program Effectiveness – What’s the Government Looking for in Your Compliance Program?” American Health Law Association (AHLA) Fraud and Compliance Forum (October 2016)
- Speaker, “Understand Trends in Recent Enforcement Targets,” CBI’s 10th Annual Forum on Transparency and Aggregate Spend (Aug. 2016)
- Speaker, “From the Boardroom to the Big House? How Corporate Compliance and Government Regulations Keep You Safe,” Memphis Bar Association (July 2016)
- Speaker, “U.S. Department of Justice Healthcare Compliance Update,” Global Healthcare 10th International Pharmaceutical Compliance Congress (May 2016)
- Speaker, “U.S. Healthcare Fraud Enforcement,” CBI’s 13th Annual Pharmaceutical Compliance Congress (April 2016)
- “Emerging Enforcement Trends in Healthcare,” American Bar Association (ABA) 30th Annual National Institute on White Collar Crime (March 2016)

June 5, 2025
Spotlight
Chambers and Partners Releases 2025 USA Guide Featuring 79 Jackson Walker Attorneys and 28 Ranked Departments
Jackson Walker is pleased to announce that Chambers and Partners has selected 79 attorneys and 28 departments for inclusion in the 2025 edition of the Global and USA guides. Attorneys were ranked across 29 departments, with 4 attorneys listed among multiple areas, 9 ranked nationwide in their respective areas, and 12 recognized for the first time.

December 16, 2024
Newsletters
JW Belonging & Inclusion Newsletter – December 2024
View Jackson Walker’s December 2024 Belonging & Inclusion Newsletter, Perspectives.

September 9, 2024
Attorney News
Jackson Walker Congratulates 36 Attorneys Named Among the Lawdragon 500 Leading Litigators in America
Jackson Walker is pleased to announce the selection of 36 attorneys to Lawdragon’s 2025 list of the 500 leading litigators in America. The third edition of this list recognizes America’s top talent in antitrust, financial and securities litigation, intellectual property, commercial litigation, real estate, mergers and acquisitions, cybersecurity, and white collar and investigations.

August 29, 2024
Speaking Engagements
Laura Kidd Cordova to Present at ABA Southeastern White Collar Crime Institute
Jackson Walker partner Laura M. Kidd Cordova will participate in a panel discussion titled “Cross Border Privilege Issues” during the 10th Annual Southeastern White Collar Crime Institute, which will take place from September 4-6, 2024.

August 28, 2024
Speaking Engagements
Laura Kidd Cordova to Present on “False Claims Act Developments” at UT School of Law Government Enforcement Institute; Jennifer Freel Serving on Planning Committee
Jackson Walker partner Laura M. Kidd Cordova will be a panelist at The University of Texas School of Law’s 10th Annual Government Enforcement Institute (UTGEI) on September 19-20. Additionally, Jackson Walker partner Jennifer S. Freel is serving on the UTGEI planning committee.
August 15, 2024
Attorney News
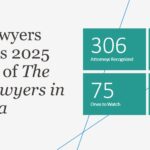
‘The Best Lawyers in America’ Features 306 Jackson Walker Attorneys in 2025 Edition, Including 9 “Lawyers of the Year” and 75 “Ones to Watch”
The Best Lawyers in America has recognized 306 Jackson Walker attorneys across 81 specialty practice areas in its 2025 edition, including 9 Lawyers of the Year and 75 Ones to Watch. In this year’s guide, Jackson Walker saw the largest number of attorneys in the areas of Commercial Litigation (75 attorneys) and Real Estate Law (55 attorneys) and the addition of 27 attorneys as Best Lawyers.

June 6, 2024
Spotlight
Women-Led Investigations & White Collar Group Recognized in Chambers USA Guide
Jackson Walker proudly announces the first-time ranking of the Investigations & White Collar practice group in the Chambers USA Guide. This ranking is anchored by the continued recognition of practice group leader Erica Benites Giese and partner Jennifer S. Freel.

April 15, 2024
Newsletters
JW Diversity & Inclusion Newsletter – April 2024
View Jackson Walker’s April 2024 Diversity & Inclusion Newsletter, Perspectives.

April 4, 2024
Speaking Engagements
Laura Kidd Cordova to Present at ABA National Institute on Health Care Fraud
Jackson Walker partner Laura M. Kidd Cordova will join a panel discussion titled “Criminal and Civil Enforcement and COVID Fraud” during the 34th Annual ABA National Institute on Health Care Fraud, taking place from May 1-3, 2024, at the InterContinental Chicago Magnificent Mile.
Practice Experience
- Represented social media influencer charged with securities fraud—all charges dismissed
- Represented foreign-exchange trader charged with wire fraud for allegedly “front-running” a foreign exchange transaction—all charges dismissed
- Represented CEO in DOJ and SEC securities fraud investigations—no charges brought
- Represented pharmacy chain in DOJ investigation related to allegations of improperly obtained prior authorizations and violations of the Anti-Kickback Statute—no charges brought
- Represented trade organization in DOJ investigation related to health insurance premium assistance programs—no charges brought
- Represented nonprofit in DOJ investigation related to pharmaceutical patient assistance programs—no charges brought
- Represented executive in DOJ investigation related to drug pricing and alleged Anti-Kickback Statute violations—no charges brought
- Represented pharmaceutical sales representative in DOJ investigation related to alleged off-label marketing and violations of the Anti-Kickback Statute—no charges brought
- Represented hospital system in DOJ and state Attorney General investigations related to tax credits—no charges brought
- Represented global agricultural commodity trading company in criminal DOJ and USDA investigation related to imported agricultural products—no charges brought
- Conducted internal investigation for national hospital system in response to internal whistleblower allegations of Anti-Kickback Statute violations and improper patient inducement, and advised system regarding disclosure obligations and potential FCA liability
- Conducted internal investigation related to billing code errors, and advised regional hospital system regarding disclosure obligations and potential FCA liability
- Conducted internal investigation and advised healthcare products distribution company regarding compliance, disclosure obligations, and potential FCA liability related to various federal government contracts, including pricing obligations, the Buy American Act, and the Trade Agreements Act
- Conducted internal investigation and advised health plan regarding compliance, disclosure obligations, and potential FCA liability related to California state contract requirements
Recognition & Accolades
- Chambers USA: America’s Leading Lawyers for Business, Ranked for Litigation: White-Collar Crime & Government Investigations (Texas), 2025
- The Best Lawyers in America (Woodward/White Inc.), Commercial Litigation; Criminal Defense: White-Collar, 2023-2025
- Lawdragon 500 Leading Litigators in America, 2023-2025
- Washington DC Super Lawyer, Super Lawyers by Thomson Reuters, 2020-2021
- Assistant Attorney General’s Award for Distinguished Service, 2015
- Health and Human Services Inspector General’s Award for Excellence in Fighting Fraud, Waste, and Abuse, 2016
- IRS Criminal Investigation’s Recognition for Outstanding Support of the IRS Criminal Investigation Mission, 2016
- FBI Director’s Certificate for Demonstrated Excellence in Successfully Prosecuting a Major Criminal Case, 2014
Publications & Speeches
- Speaker, “False Claims Act Developments,” UT Law 10th Annual Government Enforcement Institute (September 2024)
- Speaker, “Cross Border Privilege Issues,” 10th Annual Southeastern White Collar Crime Institute (September 2024)
- Speaker, “Criminal and Civil Enforcement and COVID Fraud,” 34th Annual National Institute on Health Care Fraud (May 2024)
- Author, “US Supreme Court to Decide Whether the Government Can Prove Knowledge When the Defendant’s Regulatory Interpretation Is “Objectively Reasonable”,” Jackson Walker Insights (January 2023)
- Author, “U.S. Supreme Court to Decide Standard for DOJ Dismissal of Qui Tam Cases,” Jackson Walker Insights (June 2022)
- Speaker/Moderator, “Litigation Challenges in Procurement Fraud Matters,” 10th Annual False Claims and Qui Tam Trial Institute (April 2022)
- Author, “What to Know About the Department of Justice’s Nationwide Crackdown on Healthcare Fraud,” Jackson Walker Insights (September 2021)
- Speaker, “Healthcare Enforcement and Why Effective Corporate Compliance Matters,” International Association of Defense Counsel (IADC) 2020 Midyear Meeting (February 2020)
- Speaker, “Healthcare Fraud Enforcement and Corporate Compliance,” 2019 National Asian Pacific American Bar Association (NAPABA) Convention (November 2019)
- Author, “DOJ Moves to Dismiss 10 Kickback-Related False Claims Act Complaints Against Pharmaceutical Companies,” Pratt’s Government Contracting Law Report (April 2019)
- Author, “DOJ Resets Bar for Corporate-Cooperation Credit and Shifts Emphasis in False Claims Act Cases from Individual Accountability to Monetary Recovery,” Westlaw Journal’s Government Contract (January 2019)
- Speaker, “801 Recent Developments in Individual Criminal and Civil Culpability,” Health Care Compliance Association’s (HCCA) 4th Annual Healthcare Enforcement Compliance Conference (November 2018)
- Author, “Are Government and Health Care Contracts the Exception That Swallows the Brand Memo’s Rule on FCA Enforcement?” Westlaw Journal’s Government Contract (October 2018)
- Author, “Department of Justice Leadership Previews Reforms to False Claims Act Enforcement: Significant Incentives for Cooperation and Strong Compliance,” Pratt’s Government Contracting Law Report (October 2018)
- Speaker, “Developments in Individual Criminal and Civil Culpability Post-Yates Memorandum,” American Health Law Association (AHLA) Fraud and Compliance Forum (September 2018)
- Speaker, “Deep-Dive into Reverse False Claims: Preparing for the Future of False Claims for MCOs,” American Conference Institute (ACI) Managed Care Disputes and Litigation Conference (May 2018)
- Speaker, “Pricing Governance and Anticipating Fraud and Abuse Risks in Light of the Current Pricing Debate,” American Conference Institute (ACI) 18th Annual Conference on Fraud and Abuse (March 2018)
- Quoted, “Ex-Fed. Health Care Prosecutor Laura Cordova Discusses Whistleblower Suits, Their Future Under Trump,” Corporate Counsel (March 2017)
- Speaker, “Expectations of the DOJ,” Society of Corporate Compliance and Ethics (SCCE) Boston Regional Compliance & Ethics Conference (March 2017)
- Speaker, “Developments with the False Claims Act and Qui Tam Enforcement in Light of the Escobar Decision,” American Conference Institute (ACI) 17th Annual Conference on Fraud and Abuse in the Sale and Marketing of Drugs, Devices, and Medical Technology (March 2017)
- Speaker, “A Spotlight on Recent Trends in Enforcement,” CBI’s 5th Annual Compliance Monitoring Forum (February 2017)
- Speaker, “Program Effectiveness – What’s the Government Looking for in Your Compliance Program?” American Health Law Association (AHLA) Fraud and Compliance Forum (October 2016)
- Speaker, “Understand Trends in Recent Enforcement Targets,” CBI’s 10th Annual Forum on Transparency and Aggregate Spend (Aug. 2016)
- Speaker, “From the Boardroom to the Big House? How Corporate Compliance and Government Regulations Keep You Safe,” Memphis Bar Association (July 2016)
- Speaker, “U.S. Department of Justice Healthcare Compliance Update,” Global Healthcare 10th International Pharmaceutical Compliance Congress (May 2016)
- Speaker, “U.S. Healthcare Fraud Enforcement,” CBI’s 13th Annual Pharmaceutical Compliance Congress (April 2016)
- “Emerging Enforcement Trends in Healthcare,” American Bar Association (ABA) 30th Annual National Institute on White Collar Crime (March 2016)
Practice Areas
Experience
- Employment & Governmental Investigations
- Export Controls Enforcement
- Global Anti-Corruption Compliance and Defense
- Global Corporate Victim Advocacy
- Government Contracts & Procurement
- Healthcare Fraud & Abuse
- Healthcare Litigation
- Healthcare Regulatory Compliance
- Investigations & White Collar Defense
- Securities Enforcement
- Special Investigations
